Activated carbon is a cornerstone technology in modern gold hydrometallurgy, serving as the primary medium for recovering dissolved Gold from cyanide solutions. Its application has fundamentally reshaped the economics of the gold mining industry, enabling the profitable extraction of Gold from ore bodies that were previously considered uneconomic. The core function of activated carbon is its remarkable ability to selectively adsorb the gold-cyanide complex, an anionic species formed during the leaching process, thereby concentrating it from a dilute solution onto a solid, easily separable medium.
, Carbon-in-Leach (CIL), and Carbon-in-Column (CIC) processes_.png)
Pictures are for reference only
This capability is leveraged in three principal process configurations: Carbon-in-Pulp (CIP), where carbon is contacted with the ore slurry after leaching; Carbon-in-Leach (CIL), where leaching and adsorption co-occur in the same vessels; and Carbon-in-Column (CIC), used to recover Gold from the clear solutions generated by heap leaching operations. The widespread adoption of these methods since the mid-20th Century stems from their unparalleled efficiency and their unique ability to overcome the limitations of older technologies, particularly in the treatment of low-grade and metallurgically complex ores.
However, the implementation of activated carbon circuits presents significant challenges. The technology presents a duality: on one hand, it offers exceptional recovery efficiency and process simplification; on the other, it introduces complexities related to the physical and chemical degradation of the carbon, the high capital and energy costs of the ancillary gold recovery and carbon reactivation circuits, and the rigorous environmental and safety management required.
The progression of gold extraction technology reflects a clear and logical response to the evolving nature of the ore deposits being exploited. As easily accessible, high-grade surface deposits were depleted, the industry was compelled to develop more sophisticated methods to treat lower-grade and more complex hard-rock ores, leading to the technological succession that culminated in the dominance of carbon-based processes.
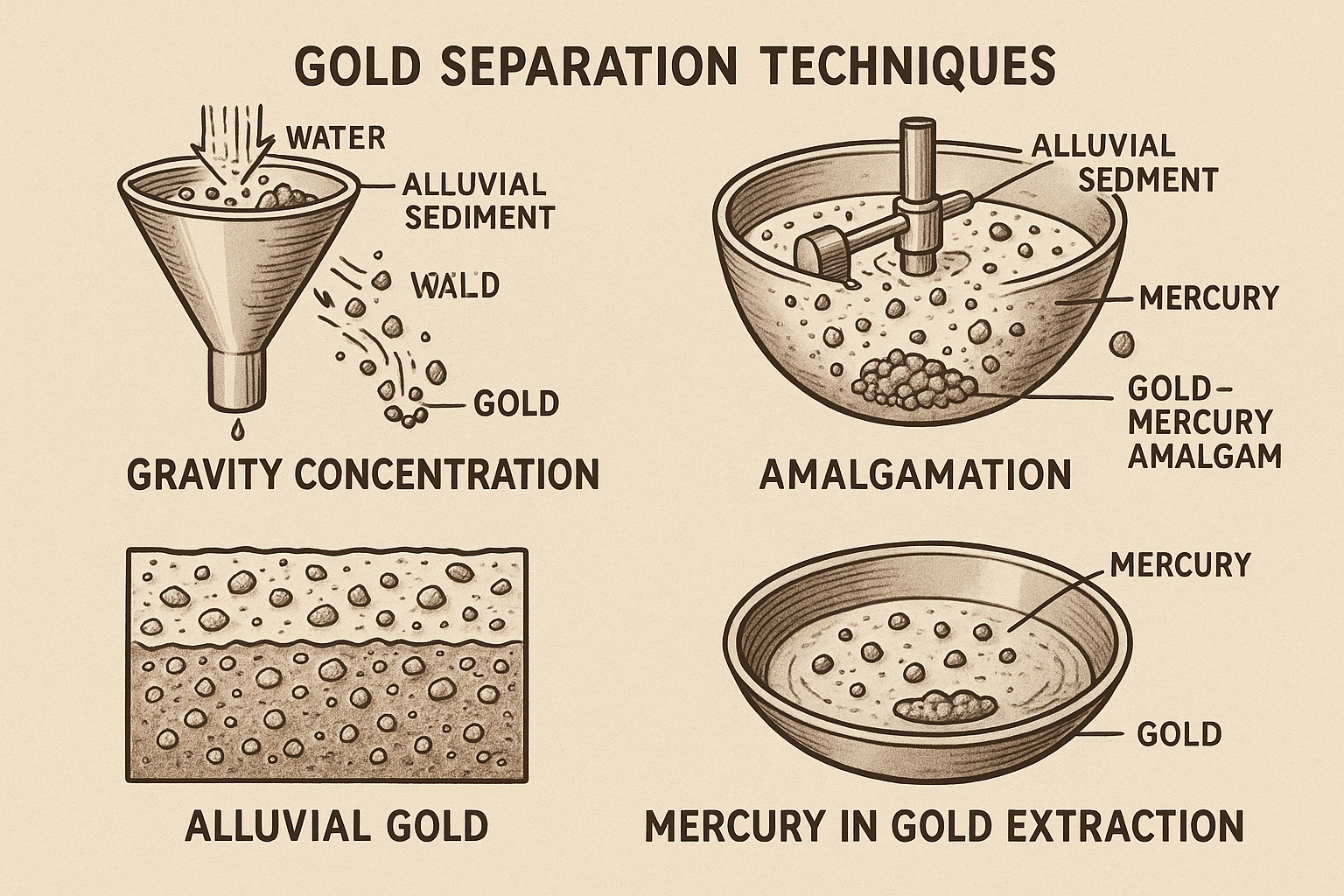
The earliest forms of gold recovery relied entirely on the unique physical properties of native, metallic Gold. Gravity concentration was the foundational method, leveraging Gold's exceptionally high specific gravity (19.3) to separate it from much lighter gangue minerals such as quartz and silicates (SG 2.8-4.0). Simple techniques, such as manual gold panning and sluice boxes, evolved into more mechanized systems, including jigs, spiral concentrators, and shaking tables, to process larger volumes of alluvial sands and gravels.
For hard-rock ores, the rock first had to be crushed to liberate the gold particles, initially with devices like the arrastre and later with more efficient stamp mills. To capture the wonderful gold particles that would otherwise be lost, gravity concentration was often paired with mercury amalgamation. In this process, a slurry of crushed ore and water was passed over mercury-coated copper plates. The Gold would dissolve into the mercury to form an amalgam, which was then periodically scraped off and heated in a retort to vaporize the mercury, leaving the purified Gold behind.
These pre-cyanidation methods, while effective for their time, suffered from a critical limitation: they could only recover coarse, physically liberated Gold, often referred to as "free-milling" Gold. They were profoundly inefficient for ores containing finely disseminated or sub-microscopic gold particles locked within the matrix of other minerals, especially sulfides. As mining exhausted the easily treated oxidized surface ores and delved into more complex primary deposits, these methods resulted in unacceptable gold losses, creating a pressing need for a chemical extraction process. Furthermore, the widespread use of mercury presented severe health and environmental hazards due to its high toxicity and the inevitable losses to air and tailings.
Pictures are for reference only
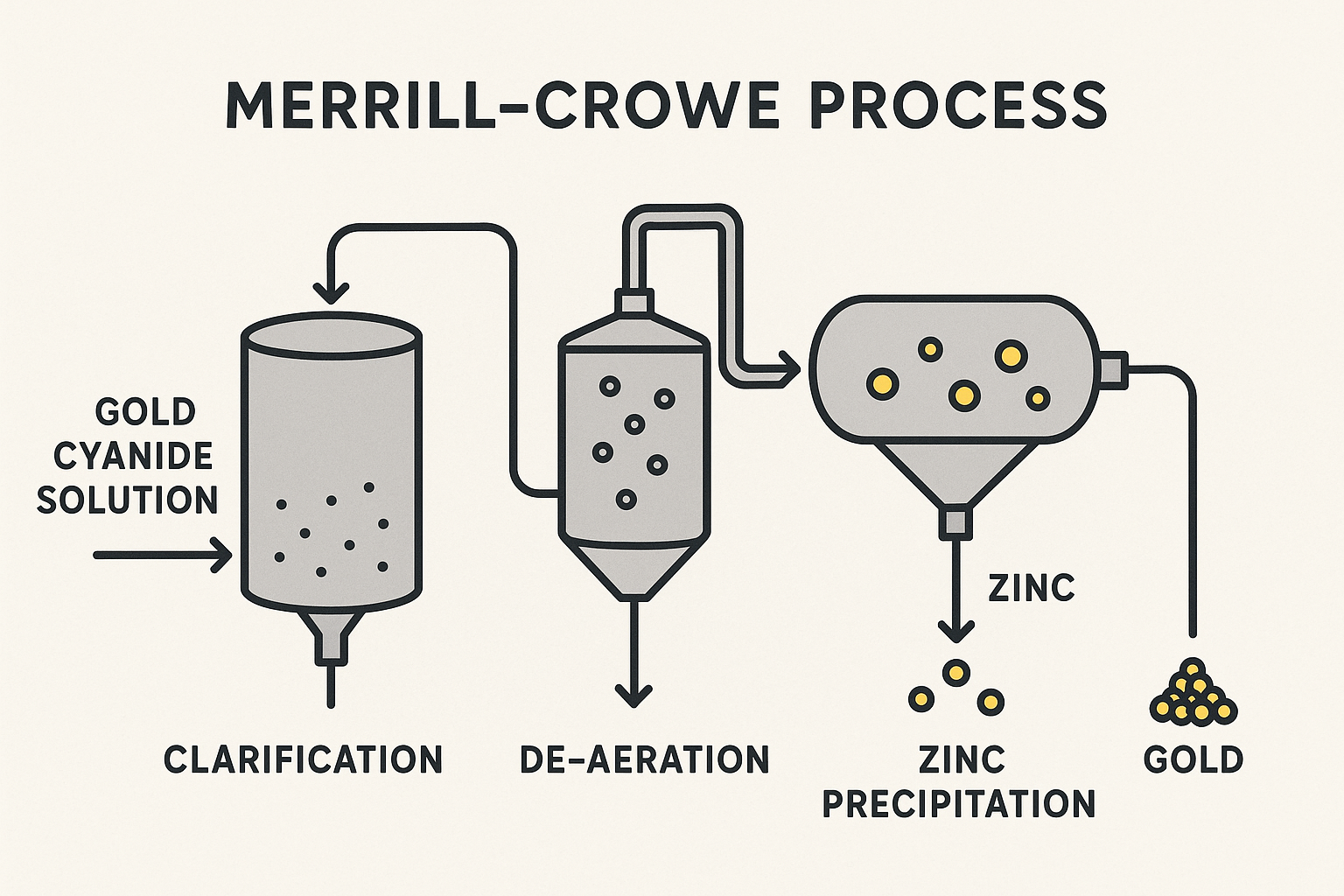
The invention of the cyanidation process in the late 19th Century was a paradigm shift in gold metallurgy. It provided a chemical means to dissolve fine and locked gold particles into an aqueous solution, making vast new ore bodies viable. For over 80 years, the preeminent technology for recovering this dissolved Gold was the Merrill-Crowe process, an enhancement of the original MacArthur-Forrest process.
The Merrill-Crowe process is predicated on a cementation reaction where Gold is precipitated from the pregnant leach solution by the addition of a more electropositive metal, typically zinc dust. The successful execution of this process is critically dependent on a sequence of carefully controlled steps:
For ores that yielded a clean, easily filterable leach solution, the Merrill-Crowe process was highly efficient and reliable, establishing itself as the global industry standard for decades, especially for operations with a high silver-to-gold ratio.
Pictures are for reference only
The dominance of the Merrill-Crowe process began to wane as the mining industry increasingly turned its attention to large, low-grade deposits and metallurgically "difficult" ores. The very strength of the Merrill-Crowe process—its reliance on a pristine, solids-free solution—became its critical weakness when faced with these new challenges.
Many large ore bodies, particularly those in weathered geological profiles, contain high percentages of clay minerals and fine silts. When ground and leached, these "slimy ores" produce a pulp with extremely poor settling and filtering characteristics. For such ores, the solid-liquid separation step required for Merrill-Crowe becomes an insurmountable technical and economic hurdle. The necessary infrastructure, including massive counter-current decantation (CCD) thickener trains and large-scale clarification and filtration plants, represents an enormous capital and operating expenditure that can render an otherwise viable low-grade deposit uneconomic.
A second major challenge emerged with the processing of carbonaceous or "preg-robbing" ores. These ores contain naturally occurring carbonaceous matter, similar to activated carbon, which can adsorb the dissolved aurocyanide complex, resulting in significant gold losses. The Merrill-Crowe flowsheet offered no practical solution to this phenomenon. These limitations created a powerful economic driver for the development of a new technology that could circumvent the solid-liquid separation bottleneck and effectively treat these increasingly important ore types.
The development of carbon-based adsorption technologies in the mid-20th Century was a direct and revolutionary response to the limitations of the Merrill-Crowe process. By enabling the recovery of Gold directly from the ore slurry, these methods eliminated the costly and problematic solid-liquid separation stage, unlocking the economic potential of vast, previously untreatable resources.
The concept of using carbon to adsorb Gold has been known since the 19th Century. However, its practical application in a pulp medium was not realized until much later. Pioneering research in the 1950s and subsequent development through the 1970s led to the first commercial Carbon-in-Pulp plants. The central innovation was the use of hard, granular activated carbon particles, typically 1-3 mm in size. These granules were large and durable enough to be mixed directly with the finely ground ore slurry (normally <75 µm). They could subsequently be separated from the pulp by simple screening, a feat impossible with the fine zinc dust used in the Merrill-Crowe process.
The development of this core concept led to two primary process configurations that now dominate the industry:
 and Carbon-in-Leach (CIL) processes showing the flow of gold recovery through cyanide leaching and adsorption_.png)
The principles of carbon adsorption were also adapted for treating clear solutions, primarily those generated from heap leaching. Heap leaching is a low-cost method used to process huge tonnages of low-grade ore, where a dilute cyanide solution is irrigated over a large pile, or "heap," of crushed ore. The resulting pregnant leach solution (PLS), which drains from the heap, is collected and pumped to a recovery plant.
The Carbon-in-Column (CIC) process is the standard method for this application. The PLS is pumped upwards through a series of large columns containing a fluidized bed of activated carbon. The up-flow, fluidized-bed design is robust and can tolerate the presence of suspended solids (up to 2-3 wt%), which are often washed out of the heap, a condition that would quickly clog the filters of a Merrill-Crowe circuit. The symbiotic relationship between low-cost heap leaching and efficient CIC recovery has enabled the development of many of the world's largest gold mines.
Table 1: A Comparative Overview of Gold Recovery Technologies | Technology | Principle of Operation | Typical Feed Ore | Key Advantages | Critical Disadvantages | |:--- |:--- |:--- |:--- |:--- | | Gravity/Amalgamation | Physical separation based on Gold's high density; collection with mercury. | Alluvial deposits; high-grade, coarse "free gold" ores. | Simple; low-cost; effective for coarse Gold. | Inefficient for fine/disseminated Gold; severe environmental/health risks from mercury. | | Merrill-Crowe | Chemical precipitation of dissolved Gold from a clear solution using zinc dust. | Ores amenable to producing a clear, solids-free leach solution. | High recovery from clear solutions; effective for high-silver ores. | Requires complete solid-liquid separation; high CAPEX for thickeners/filters; cannot treat "slimy" ores. | | CIP / CIL | Adsorption of dissolved Gold directly from ore slurry onto activated carbon granules. | Low-grade ores; "slimy" ores with high clay/fines; "preg-robbing" carbonaceous ores. | Eliminates solid-liquid separation; lower CAPEX; effective for difficult ores. | Complex back-end circuit (elution/regeneration); carbon attrition and gold loss; gold inventory lock-up. | | CIC | Adsorption of dissolved Gold from a clear/semi-clear solution onto carbon in columns. | Low-grade heap leach solutions. | Efficiently concentrates Gold from very dilute solutions; tolerant of some suspended solids. | Limited to solutions, not applicable to slurries. |
The entire technological framework of modern gold hydrometallurgy rests on a two-step chemical sequence: the dissolution of solid Gold into an aqueous complex, followed by the recovery of that complex from the solution.
The dissolution of Gold in a cyanide solution is an oxidative process first described by Elsner in the 19th Century. The reaction, represented by Elsner's Equation, requires four components: metallic Gold (Au), cyanide ions (CN−), an oxidant (dissolved oxygen, O2), and water (H2O). The process is conducted in an alkaline environment (typically pH 10.5-11) to ensure the cyanide remains in its ionic form and to prevent the evolution of highly toxic hydrogen cyanide (HCN) gas. The overall balanced chemical reaction is:
2Au(s)+4CN(aq)−+O2(g)+2H2O(l)→2[Au(CN)2](aq)−+2OH(aq)−
This reaction transforms the solid, elemental Gold into the dicyanoaurate(I) anion, a highly stable and soluble complex ion. It is this transformation that allows Gold to be mobilized from the solid ore into the liquid phase, from which it can be recovered and concentrated.
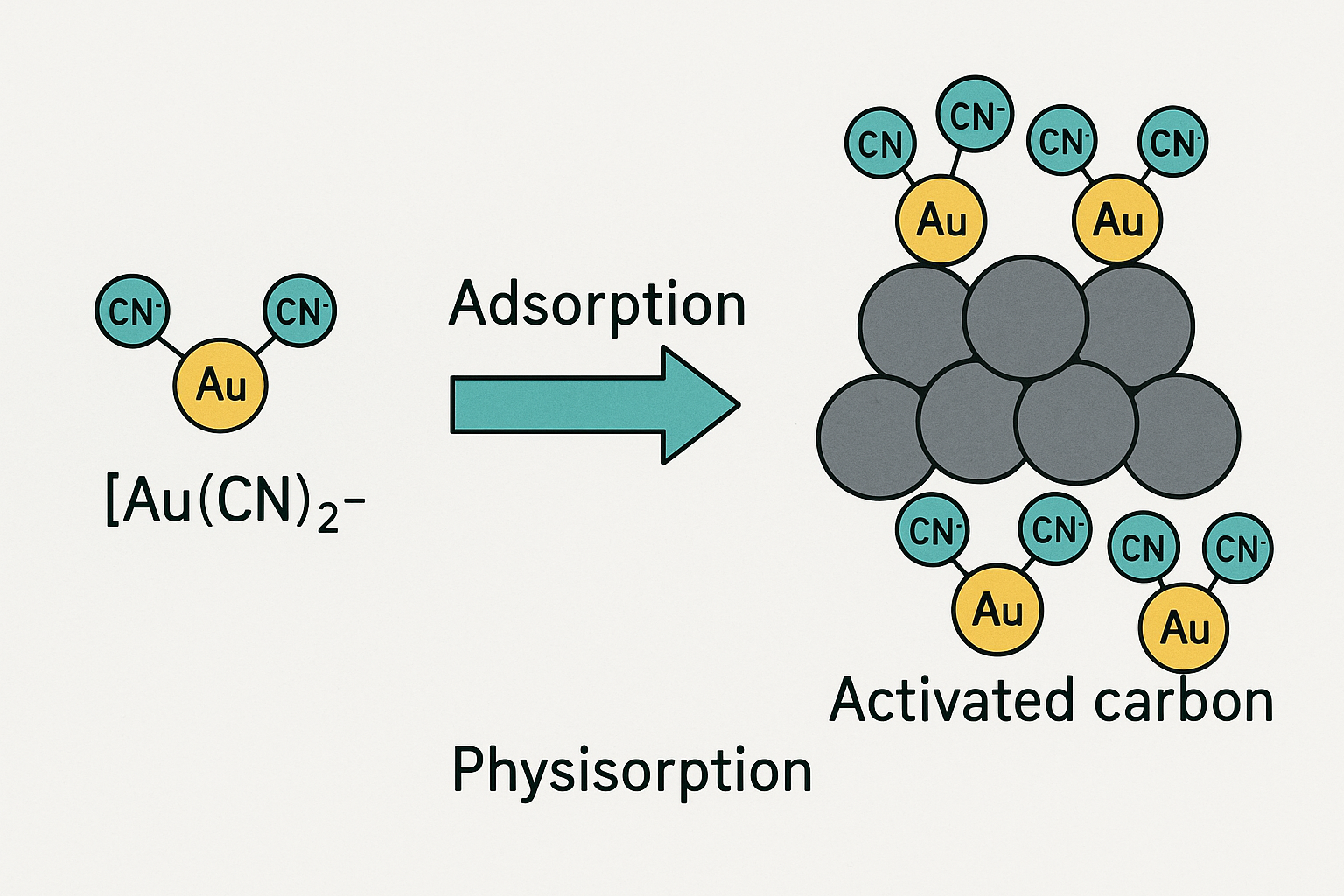
Once in solution, the aurocyanide complex is recovered by adsorption onto the surface of activated carbon. While the exact mechanism has been a subject of extensive research, the most widely accepted theory involves the formation and subsequent adsorption of a neutral ion pair.
The negatively charged aurocyanide anion, [Au(CN)2]−, does not readily adsorb onto the largely neutral surface of the activated carbon by itself. However, process solutions contain various cations, such as sodium (Na+), potassium (K+), and particularly calcium (Ca2+), which is often present from the lime added for pH control. These cations associate with the aurocyanide anion to form a neutral or less-charged ion pair, such as Ca2+[Au(CN)2]2−. This neutral complex can then be readily adsorbed onto the non-polar graphitic surfaces within the carbon's micropores via physisorption (physical adsorption) mechanisms, likely driven by van der Waals forces. The adsorption process is an equilibrium reaction and is exothermic, meaning it is reversible and is favored at lower temperatures. This key principle is exploited in the high-temperature elution step to recover the Gold. The presence of divalent cations like Ca2+ has been shown to significantly enhance the rate and strength of adsorption compared to monovalent cations like Na+.
Pictures are for reference only
While activated carbon has revolutionized gold extraction by enabling the economic processing of diverse ore types, its application is a complex engineering trade-off, balancing unparalleled recovery efficiency against inherent challenges in material degradation, process complexity, and rigorous environmental management.
The ascendancy of activated carbon in gold hydrometallurgy is rooted in its unique combination of physical and chemical properties. These attributes translate directly into significant operational and economic efficiencies, particularly when compared to the Merrill-Crowe technology that preceded it. The case for activated carbon rests on its superior adsorption characteristics, its ability to simplify the plant flowsheet, and its unmatched performance with ores that are otherwise difficult or impossible to treat.
The effectiveness of activated carbon is a direct result of its engineered physical structure and the chemical interactions that occur at its surface. These intrinsic properties allow it to selectively capture and concentrate Gold from complex solutions with remarkable efficiency.
The performance of activated carbon begins with the selection of its raw material and the highly controlled manufacturing process that transforms it into a powerful adsorbent.
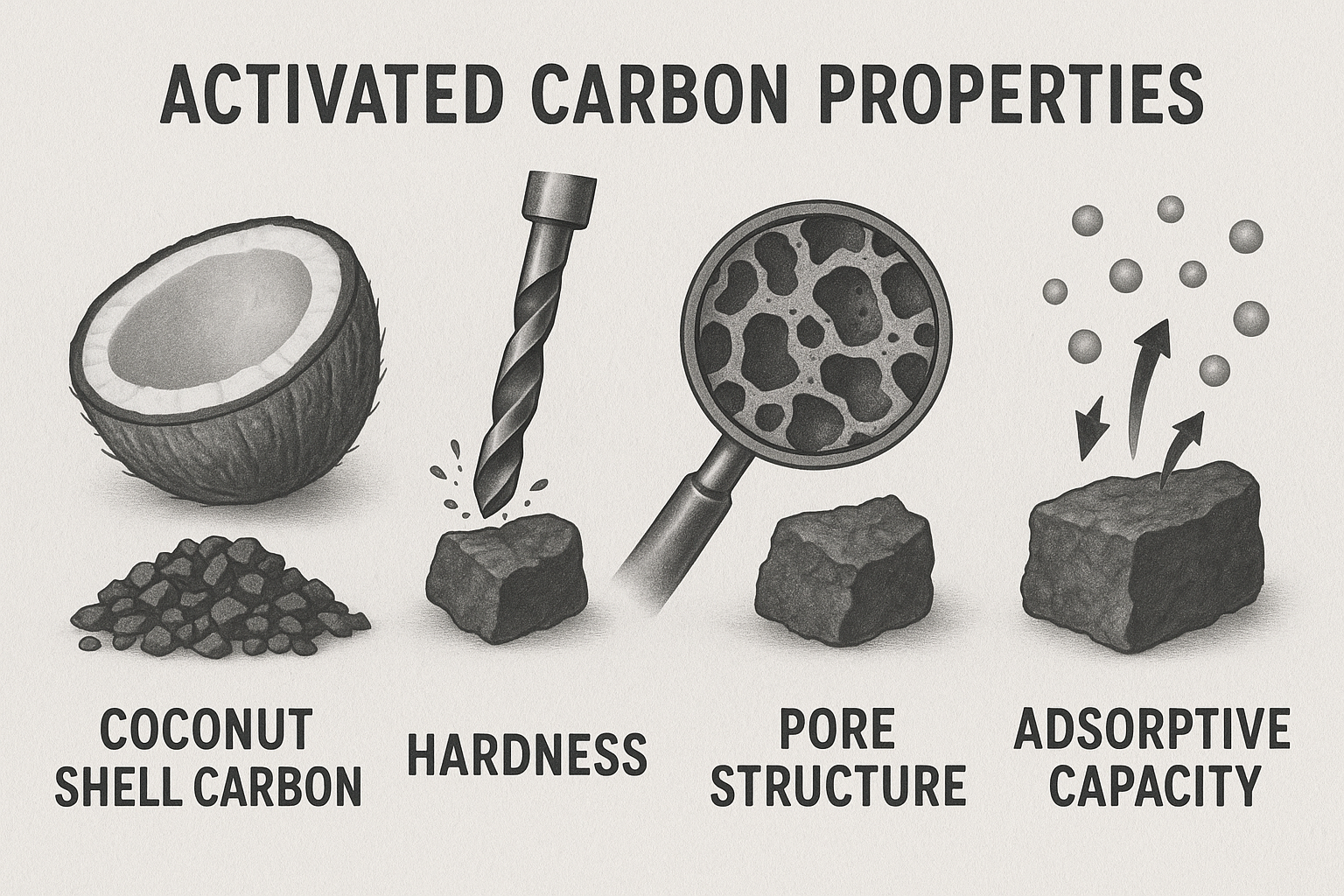
Activated carbon can be produced from a variety of carbonaceous feedstocks, including coal, wood, and various nutshells. For the demanding application of gold recovery in CIP and CIL circuits, coconut shell is the universally preferred raw material. The selection of coconut shell is not arbitrary; its inherent lignocellulosic structure yields a final product with a superior combination of properties. Specifically, coconut shell-based carbon exhibits exceptional hardness and resistance to Attrition, which is critical for surviving the abrasive slurry environment of agitated tanks. Furthermore, the activation of coconut shell produces a carbon with a highly developed network of micropores, the ideal size for adsorbing the relatively small gold-cyanide complex. Carbons derived from coal or wood are typically softer and possess a different pore size distribution, with a greater proportion of larger pores, making them less durable and less efficient for this specific application.
Table 2: Properties of Activated Carbon by Raw Material Source for Gold Recovery | Raw Material | Key Characteristics | Typical Hardness (% Ball Pan) | Dominant Pore Structure | Suitability for CIP/CIL | |:--- |:--- |:--- |:--- |:--- | | Coconut Shell | Tough, high density, attrition resistant, high activity. | >98% | Highly Microporous (<2nm) | Excellent | | Bituminous Coal | Moderate hardness, lower density. | Variable, generally lower than coconut. | Mesoporous (2-50nm) | Moderate; higher attrition risk. | | Wood / Peat | Soft, low density, low abrasion resistance. | Low | Macroporous (>50nm) | Poor; unsuitable for abrasive slurry applications. |
Pictures are for reference only
The transformation of raw coconut shell into activated carbon is a two-stage thermal process.
The efficacy of activated carbon is not just a function of total surface area, but also of the distribution of pore sizes within each granule. This complex network is a transport system designed for efficient adsorption.
This hierarchical pore structure is essential for performance. Without the larger macro- and mesopores, diffusion into the particle would be extremely slow, resulting in poor kinetics. Without the extensive micropore network, the carbon would lack the high capacity needed for economic gold loading.
The chemical and physical interactions between the gold complex and the carbon surface are what drive the recovery process. These interactions are characterized by high selectivity, capacity, and speed.
Gold leach solutions are rarely pure; they often contain significant concentrations of other metals that also form cyanide complexes, such as copper, zinc, nickel, and iron. A critical advantage of activated carbon is its strong preferential affinity for the aurocyanide complex over these competing base metal complexes. While some co-adsorption of metals, such as copper, does occur, Gold is loaded to a much greater extent. This selectivity enables the effective concentration of Gold even from chemically complex solutions, simplifying the subsequent elution and refining steps and improving the purity of the final gold product.
The ultimate amount of Gold that carbon can hold at equilibrium is a fundamental measure of its quality. This relationship is quantified by an adsorption isotherm, which plots the amount of Gold on the carbon against the concentration of Gold remaining in the solution. For gold recovery, the Freundlich isotherm model is often used to describe this behavior. From this data, the industry derives a key performance indicator known as the K-value. The K-value is defined as the theoretical gold loading on the carbon (typically in mg/g or kg/t) when it is in equilibrium with a solution containing 1 mg/L (1 ppm) of Gold. A higher K-value signifies a greater loading capacity, meaning less carbon is required to recover the same amount of Gold, which has significant implications for the size and cost of the downstream elution and regeneration circuits.
In a continuous industrial process, such as CIP or CIL, the speed of adsorption is as essential as the ultimate capacity. The rate at which carbon loads gold is known as its adsorption kinetics. This process is governed by mass transfer, involving the diffusion of the gold complex through the liquid film surrounding the carbon particle and then through the internal pore structure. To provide a standardized measure of this property, the industry uses the R-value. The R-value represents the percentage of Gold that a sample of carbon can adsorb from a standard gold solution under controlled conditions in a specified time, typically one hour. A high R-value indicates fast kinetics, which translates to higher stage efficiencies in the plant, allowing for either shorter residence times or lower soluble gold losses for a given circuit configuration.
The superior physical and chemical properties of activated carbon directly result in tangible metallurgical and economic benefits at the plant scale.
The powerful combination of high loading capacity (K-value) and rapid adsorption kinetics (R-value) allows carbon circuits to operate with exceptional efficiency. In a well-designed and properly managed circuit, it is common to achieve over 99% recovery of the dissolved Gold from the pregnant leach solution.
This high recovery efficiency directly translates into one of the most critical plant performance metrics: the concentration of soluble Gold in the final tailings stream. Efficient carbon circuits can reduce the soluble gold tenor to extremely low levels, often below 0.01 mg/L. Minimizing these soluble losses is paramount, as every gram of Gold lost to tailings is a direct loss of revenue.
The most significant impact of activated carbon has been its role in making low-grade mining viable. Many of the world's largest gold deposits have average grades of less than 1-2 grams of gold per tonne of ore (g/t). The cyanidation of these ores produces vast quantities of very dilute pregnant solutions. Activated carbon's ability to efficiently scavenge and concentrate Gold from these extremely low concentrations is the key technological enabler. It can focus the Gold by a factor of 1,000 to 1,500 times, from a few parts per million in solution to several thousand grams per tonne on the loaded carbon. Without this concentrating power, the cost of recovering such small amounts of Gold would be economically prohibitive.
Beyond its intrinsic adsorptive properties, the use of activated carbon in a pulp medium (CIP/CIL) offers significant operational and economic advantages over the Merrill-Crowe process, which it largely replaced. These benefits stem from a radical simplification of the process flowsheet.
The single most significant advantage of the CIP and CIL processes is the elimination of the entire solid-liquid separation circuit that is mandatory for the Merrill-Crowe process.
By recovering Gold directly from the ore slurry, carbon-based circuits bypass the most capital-intensive and operationally challenging section of a traditional cyanidation plant.
The elimination of the entire solid-liquid separation train yields substantial project benefits. The overall plant footprint is significantly smaller, resulting in reduced civil engineering and construction costs. The reduced equipment count leads to faster construction and commissioning schedules. Most importantly, the total initial capital expenditure (CAPEX) for a CIP/CIL plant is substantially lower than for a Merrill-Crowe plant of the same throughput capacity, making it a more financially attractive option for many mining projects.
The operational simplicity of the front-end of a carbon circuit also translates into lower ongoing operating costs.
Activated carbon is a reusable reagent. After the Gold is recovered from the loaded carbon in the elution circuit, the now-barren carbon is not discarded. It is thermally reactivated in a regeneration kiln to restore its adsorptive properties. It is then returned to the adsorption circuit. This continuous recycling loop significantly reduces the consumption rate of new ("virgin") carbon, thereby reducing a significant operational cost. This reusability is a key advantage over the single-use zinc dust consumed in the Merrill-Crowe process.
The operation and maintenance of a CIP/CIL adsorption circuit, which primarily consists of agitated tanks, pumps, and screens, is far simpler than managing a complex train of thickeners, clarifiers, and filter presses. This results in lower requirements for operational labor and reduced maintenance costs associated with mechanically complex equipment.
The operational model of contacting carbon directly with slurry makes CIP/CIL uniquely capable of handling ores that are metallurgically challenging or impossible for the Merrill-Crowe process.
This is the quintessential advantage that drove the adoption of carbon-based technologies. Ores with high concentrations of clay minerals or other naturally fine particles produce slurries with high viscosity and very slow settling rates. Attempting to filter such a slurry in a Merrill-Crowe circuit would result in the rapid blinding of the filter media, rendering the process inoperable. In a CIP or CIL circuit, these fine particles remain in the slurry and pass through the inter-tank screens, posing no fundamental obstacle to the adsorption process. The development of CIP/CIL directly enabled the economic processing of these widespread "slimy" ore types.
Carbon-based technology, particularly the CIL configuration, provides the most effective and widely used solution for overcoming the challenge of preg-robbing ores.
While the adoption of activated carbon technology solved critical problems in gold hydrometallurgy, it introduced a new suite of complex engineering, operational, and financial challenges. The simplicity gained by eliminating the solid-liquid separation stage is offset by the intricacy of managing the carbon itself and the necessity of a sophisticated "back-end" circuit to recover the Gold and regenerate the carbon. These disadvantages represent the other side of the engineering trade-off. They are central to the daily operation and economic optimization of a modern gold plant.
Activated carbon, despite its hardness, is a consumable material that physically degrades within the harsh, abrasive environment of a CIP or CIL circuit. This degradation, known as Attrition, is a primary operational concern as it leads directly to the loss of both carbon and the valuable Gold it carries.
.png)
Pictures are for reference only
Carbon granules are subjected to continuous mechanical stress from the moment they enter the adsorption circuit until they are removed for regeneration. This stress manifests through two primary mechanisms:
The cumulative effect of abrasion and impact is the generation of tiny carbon particles and dust, collectively known as carbon fines. The loss of these fines from the circuit is a significant source of irrecoverable gold loss. A crucial aspect of this problem is that gold adsorption is predominantly a surface phenomenon, occurring on the outer layers of the carbon particle. Consequently, the fine particles abraded from the surface have a disproportionately high gold loading compared to the core of the parent granule, making their loss particularly costly.
To facilitate the counter-current flow of pulp and carbon, each adsorption tank is fitted with inter-tank screens. These are typically robust wedge-wire or polyurethane panels with precisely sized apertures (e.g., 0.7-0.8 mm) designed to retain the relatively coarse activated carbon granules (typically 1-3 mm) within the tank while allowing the much finer ore pulp (typically <0.1 mm) to pass through to the subsequent tank.
While the screens are effective at retaining the bulk of the carbon inventory, they are unable to capture the fine carbon particles generated through Attrition. Any carbon fine smaller than the screen aperture will pass through with the slurry. These gold-laden fines are progressively washed down the tank train. They are ultimately discharged from the circuit with the final tailings slurry. This represents a direct and permanent loss of revenue. Total carbon losses in a plant can be significant, often ranging from 40 to 65 grams of carbon per tonne of ore processed, with Attrition in the adsorption and regeneration circuits being the primary contributors.
Given the significant economic impact of Attrition, both quantifying the durability of carbon and designing circuits to minimize its degradation are critical.
The primary strategy for mitigating Attrition is the selection of a physically robust carbon. The mining industry relies on standardized laboratory tests to measure carbon hardness and predict its in-circuit performance. The most widely accepted method is the Ball Pan Hardness test (ASTM D3802). In this test, a screened sample of carbon is placed in a special pan with steel balls and subjected to a defined period of shaking and tapping. The hardness number is calculated based on the percentage of the original sample that is retained on a smaller-aperture sieve after the test. A high hardness number, typically greater than 98%, is a key purchasing specification for carbon destined for CIP/CIL applications.
The economic consequences of carbon attrition are severe. Firstly, there is the direct operational cost of purchasing new virgin carbon to replace the amount lost. Secondly, and more significantly, there is the value of the Gold adsorbed onto the lost fines, which reduces the overall plant recovery efficiency. These combined costs can dramatically impact the profitability of a mining operation, making attrition management a key focus for process engineers.
The simplicity of the CIP/CIL adsorption stage is balanced by the complexity of the "back-end" of the plant. This section, which comprises elution, regeneration, and electrowinning, is effectively a self-contained chemical processing facility. High capital costs, significant energy and reagent consumption, and complex operational control are characteristic of it.
Once the carbon is loaded with Gold to an economic level, the Gold must be recovered in a concentrated form. This is achieved through the process of elution, also known as stripping.
Elution is the reverse of adsorption. The process manipulates chemical and physical conditions to make the adsorption of the gold-cyanide complex unfavorable, forcing it to desorb from the carbon surface and return to solution. The key drivers for elution are high temperature, high cyanide strength, and high caustic (hydroxide) concentration. By increasing the temperature and the concentration of competing CN− and OH− ions in the eluting solution, the adsorption equilibrium is shifted strongly in favor of desorption.
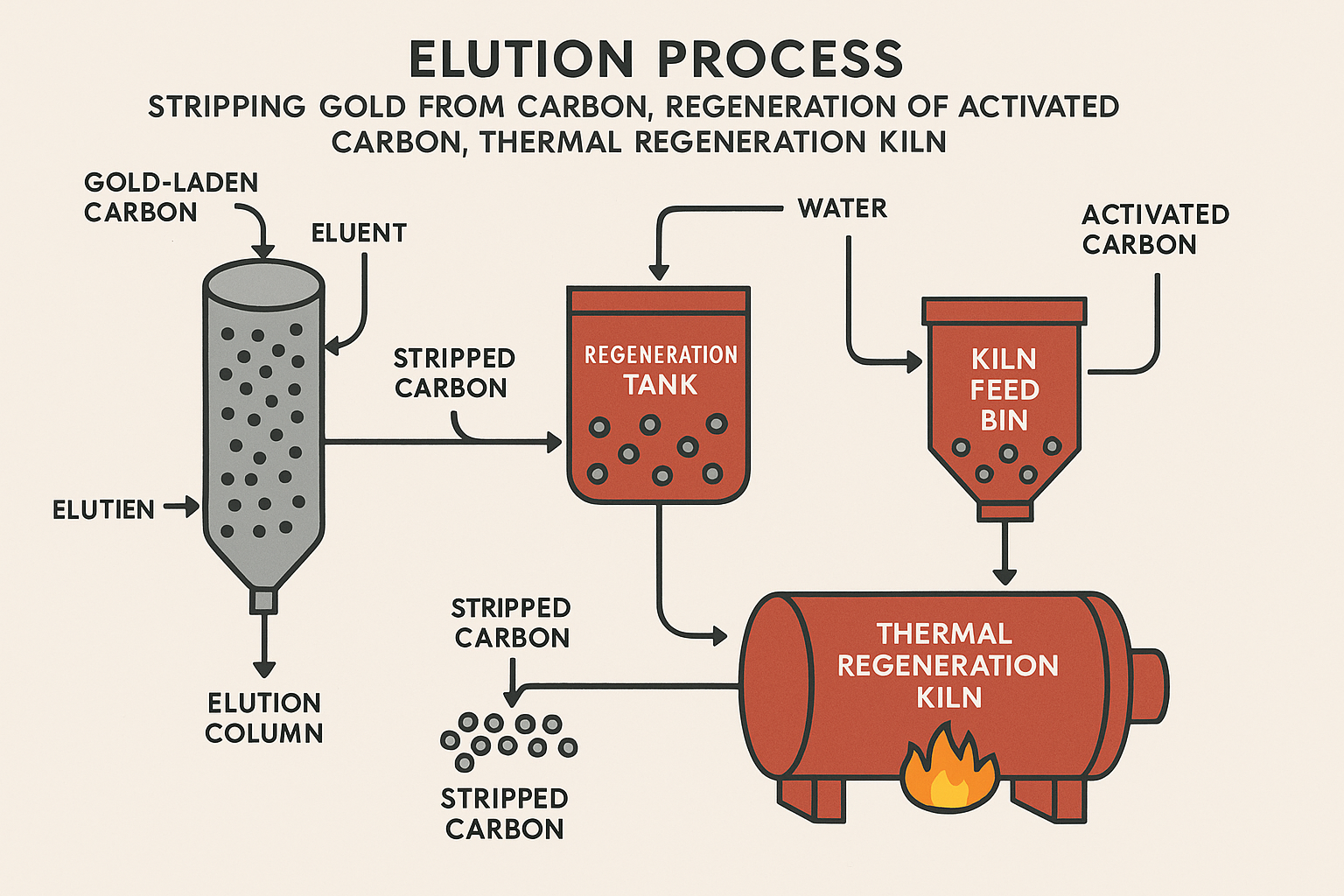
Pictures are for reference only
Two main industrial processes are used, differing primarily in their operational philosophy and reagent scheme:
Elution circuits are capital-intensive. They require insulated, high-pressure-rated steel columns to contain the carbon and hot solutions. Significant infrastructure is needed for heating the eluant, including high-power electric heaters or steam-fed heat exchangers. A system of pumps is required to circulate the hot, corrosive fluids, and extensive instrumentation is needed to control temperature, pressure, and flow rates.
The elution process is a significant utility consumer. The high temperatures required make it one of the most energy-intensive parts of the entire gold plant. It also consumes substantial quantities of high-purity reagents, including caustic soda and sodium cyanide, as well as high-quality, softened water to prevent the formation of scale on heat exchanger surfaces and within the elution column, which would severely impede performance.
After multiple cycles of adsorption and elution, the carbon's performance degrades due to the accumulation of organic foulants. Thermal regeneration is an essential, energy-intensive step to restore the carbon's activity.
While elution removes the Gold, it does not effectively remove strongly adsorbed organic compounds (e.g., lubricants, flotation reagents, humic acids). These organic "poisons" block the carbon's micropores, reducing its capacity and kinetic activity. The purpose of regeneration is to use high temperatures to pyrolyze (thermally decompose) and burn off these organic foulants, thereby clearing the pores and reactivating the carbon surface for reuse.
The heart of the regeneration circuit is the kiln, typically a horizontal, indirectly fired rotary kiln or a vertical furnace. The kiln and its associated feed and cooling systems represent a significant capital cost. Due to the high temperatures required (up to 800°C), it is also a critical energy consumer, typically fueled by electricity, diesel, or natural gas.
The regeneration process within the kiln occurs in distinct temperature zones:
Regeneration is a delicate balancing act. Suppose the temperature is too low or the residence time too short. In that case, the organic foulants will not be removed entirely, and the carbon will return to the circuit with reduced activity. Conversely, if the temperature is too high or the atmosphere is too oxidizing, the steam and oxygen will begin to react with the base structure of the activated carbon itself. This "over-burning" permanently destroys the microporous network, reduces the carbon's surface area, and can physically weaken the granules, making them more susceptible to Attrition in the future.
The final steps involve recovering the Gold from the concentrated eluate solution and converting it into a saleable product.
The gold-rich eluate is pumped to electrowinning cells. These cells contain anodes (typically stainless steel mesh) and cathodes made of a high-surface-area material, most commonly steel wool. A direct electrical current is passed through the solution. The negatively charged aurocyanide complex, [Au(CN)2]−, migrates to the positive anode. However, the electrochemical reaction causes the gold ions to be reduced to metallic Gold, which plates onto the surface of the negative cathode.
After a sufficient amount of Gold has been plated, the steel wool cathodes are removed from the cells. The gold-laden steel wool is then mixed with a blend of fluxes (such as borax, silica, and soda ash) and charged into a high-temperature smelting furnace. The fluxes combine with the steel wool and other impurities (like co-deposited copper and silver) to form a molten slag layer. The much denser molten gold and silver sink to the bottom of the furnace. This molten metal is then tapped from the furnace and poured into molds to create semi-pure bars of a gold-silver alloy known as Doré. These bars are then shipped to an external, specialized refinery for final purification.
Beyond the acute physical breakdown from Attrition, activated carbon in a gold recovery circuit is subject to a continuous process of chemical degradation, commonly referred to as "fouling." This progressive loss of activity is caused by the accumulation of both inorganic and organic species that block the carbon's porous structure, reducing its efficiency and necessitating energy-intensive rejuvenation steps, such as acid washing and thermal regeneration.
Inorganic fouling is primarily the result of mineral scale precipitation on the external surfaces of the carbon granules.
The operating conditions of a CIP/CIL circuit are highly conducive to the formation of calcium carbonate scale. Lime (Ca(OH)2) is continuously added to the slurry to maintain the high pH required for cyanidation, creating a calcium-rich environment. At the same time, air is sparged into the tanks to provide the oxygen for leaching, which also introduces carbon dioxide (CO2). The CO2 dissolves and reacts with the alkaline solution to form carbonate ions (CO32−), which then combine with the calcium ions to precipitate as insoluble calcium carbonate (CaCO3). The surface of the activated carbon serves as an ideal nucleation site for this precipitation, leading to the gradual accumulation of a hard mineral scale.
The precipitated CaCO3 scale forms a physical barrier, or "armor," on the outer surface of the carbon granules. This layer progressively blocks the entrances to the macro- and mesopores, which are the essential pathways for the aurocyanide complex to reach the high-surface-area micropores inside the particle. This physical blockage severely impedes mass transfer, resulting in a dramatic reduction in the carbon's adsorption kinetics (a lower R-value). Although the internal capacity may still be high, the gold complex cannot reach the active sites quickly enough, resulting in lower gold loadings and higher soluble gold losses in the tailings.
Inorganic fouling is effectively managed by periodically washing the carbon with a dilute acid solution. The loaded carbon is typically treated with a 3-5% solution of hydrochloric acid (HCl) before the elution step. The acid readily dissolves the calcium carbonate scale according to the reaction:
CaCO3(s)+2HCl(aq)→CaCl2(aq)+H2O(l)+CO2(g)
This process reopens the pore entrances, restoring the carbon's kinetic performance and also improving the efficiency of the subsequent elution step by exposing more of the loaded Gold.
Organic fouling is the adsorption of unwanted organic molecules, which often bind irreversibly within the carbon's micropores and compete directly with Gold for active sites.
A wide variety of organic species can be present in a gold processing circuit and act as carbon foulants. Their sources are diverse and include:
Unlike inorganic scales, which form on the exterior, organic foulants are typically smaller molecules that penetrate deeply into the carbon structure and adsorb within the micropores. They compete directly with the aurocyanide complex for the most active adsorption sites. Because they often adsorb very strongly, they are not removed during the elution process. This irreversible occupation of active sites progressively reduces the carbon's adequate loading capacity (K-value) and its kinetic activity (R-value) over successive cycles.
The primary method for removing organic foulants is thermal regeneration. The high temperatures (700-800°C) in the regeneration kiln are sufficient to thermally decompose and drive off most of the adsorbed organic compounds. This process is crucial for sustaining the long-term stability of the carbon inventory. However, the regeneration process is not perfectly efficient. Over many cycles, a gradual, permanent build-up of residual carbonaceous material (char) can occur, leading to a slow but irreversible decline in the carbon's performance.
The operation of a carbon-based gold recovery circuit is subject to a framework of broader considerations that extend beyond the immediate process metallurgy. These include stringent requirements for managing hazardous materials, controlling atmospheric emissions, and addressing the significant financial implications of the process.

Pictures are for reference only
The entire process is fundamentally based on the use of cyanide. This highly toxic chemical requires meticulous management to ensure worker safety and environmental protection.
Sodium cyanide is acutely toxic if ingested, inhaled, or absorbed through the skin. Consequently, gold mining operations must implement rigorous safety protocols for their transport, storage, and handling of Gold. To promote and standardize these practices globally, the mining industry has widely adopted the International Cyanide Management Code. This is a voluntary program that provides a comprehensive management system and certifies operations through independent third-party audits, covering all aspects of the cyanide life cycle from manufacture to disposal.
The tailings slurry discharged from the final adsorption tank contains residual free and complexed cyanide species that are toxic to aquatic life. Before this slurry can be sent to the tailings storage facility, it must be treated to reduce the cyanide concentration to environmentally compliant levels. One of the most common and effective detoxification methods is the INCO SO₂/Air process. This process involves treating the slurry with a mixture of sulfur dioxide (SO2) and air in the presence of a soluble copper catalyst. The process oxidizes the toxic Weak Acid Dissociable (WAD) cyanides to the far less poisonous cyanate (OCN−) species.
The high-temperature thermal regeneration kiln is a significant point source for atmospheric emissions that must be controlled.
Many gold ores contain trace amounts of mercury, which is co-leached with the Gold and strongly adsorbs onto the activated carbon. During thermal regeneration, this mercury is volatilized. If not captured, it will be released into the atmosphere as a toxic pollutant. Additionally, the combustion of fuel to heat the kiln produces greenhouse gases (CO2) and, depending on the fuel's sulfur and nitrogen content, can also generate sulfur oxides (SOx) and nitrogen oxides (NOx).
To comply with air quality regulations and international agreements, such as the Minamata Convention on Mercury, regeneration kilns must be equipped with off-gas treatment systems. These systems typically include a condenser or cooling circuit to capture and recover the volatilized mercury, followed by a wet scrubber or other abatement technology to remove particulate matter and other harmful gases. These systems add to both the capital and operating costs of the regeneration circuit.
A significant, often underappreciated, financial aspect of carbon circuits is the large amount of gold inventory that is continuously tied up within the process.
At any given moment in a continuously operating plant, a substantial quantity of Gold is not in a final, saleable form. This "locked-up" gold inventory is distributed throughout the circuit, as dissolved Gold in the slurry within the leach and adsorption tanks, and, most significantly, as adsorbed Gold on the entire mass of activated carbon that circulates through the adsorption, elution, and regeneration stages. The total value of this in-process inventory can easily amount to millions of dollars.
From a financial perspective, this locked-up inventory represents non-productive working capital. The revenue from this Gold is delayed until the entire cycle is completed and the Gold is smelted into a doré bar. In the financial evaluation of a new mining project, the initial investment required to fill the circuit with this gold inventory is treated as a capital cost, which negatively impacts the project's overall Net Present Value (NPV) and cash flow profile. Consequently, a key objective in circuit design and optimization is to minimize the total carbon inventory and the residence time of Gold within the circuit, thereby reducing the amount of capital tied up.
The successful operation of a gold recovery plant using activated carbon hinges on the effective management of the complex interplay between its advantages and disadvantages. Optimization is not about maximizing a single parameter but about achieving the most profitable and sustainable balance across the entire system. This requires a holistic approach that integrates strategic material management with sound engineering design and a forward-looking perspective on technological innovation.
Optimizing a carbon circuit involves a continuous cycle of monitoring, control, and strategic decision-making aimed at maximizing gold recovery while minimizing costs and mitigating the inherent challenges of the process.
The activated carbon itself is the most critical component of the circuit, and its management is paramount. This extends beyond simply adding new carbon to replace losses.
The optimization process begins before the carbon even enters the plant. The selection of new (virgin) carbon should be based on a rigorous evaluation of its key performance indicators (KPIs) and their suitability for the specific ore and plant conditions. A balance must be struck between:
The relative importance of these factors will vary; for instance, a plant processing a particularly abrasive ore may prioritize hardness, while a plant with a high gold grade may prioritize capacity.
Effective control of the circuit requires knowing the exact locations of the carbon and Gold at all times. This necessitates a robust carbon inventory management system. Regular and accurate sampling of the carbon concentration (in grams per liter of pulp) and the gold loading on the carbon in each adsorption tank is crucial. This data enables operators to maintain the desired carbon profile across the circuit, control the carbon advance rate, and provide an accurate accounting of the gold "lock-up" for financial reporting purposes. The development of automated sampling and analysis systems, such as the Carbon Scout, holds significant potential to improve the accuracy and timeliness of this critical data.
The circulating carbon inventory must be treated as a dynamic asset whose quality degrades over time. A routine monitoring program is essential to track the "health" of the carbon. This involves regularly sampling the regenerated carbon being returned to the circuit and testing its kinetic activity against a standard of virgin carbon. A drop in activity below a target threshold (e.g., 80% of virgin activity) indicates a problem in the poison-removal stages—either inefficient acid washing or inadequate thermal regeneration. This data-driven approach enables the targeted optimization of these circuits. It informs the decision on when a portion of the carbon inventory has become too degraded and must be discarded.
The physical design of the circuit components plays a significant role in mitigating the challenges of carbon attrition and loss.
The high-shear environment in adsorption tanks is a primary cause of carbon attrition. Modern plant design seeks to minimize this by using large, low-shear agitator impellers that are designed to suspend the carbon and ore particles with the minimum required energy input. Controlling the agitator speed is also critical; it should be just high enough to prevent "sanding" (the settling of coarse particles and carbon at the bottom of the tank) without creating excessive turbulence that accelerates abrasion. The design of the tank base and slurry offtakes can also be optimized to improve mixing efficiency and eliminate dead zones where carbon can accumulate.
The inter-tank screens are the primary defense against the loss of the bulk carbon inventory. The use of durable, abrasion-resistant screen materials, such as wedge-wire steel or polyurethane, is standard practice. Efficient screen cleaning mechanisms, such as mechanical wiper blades or air sparging systems, are essential to prevent the screen apertures from becoming blocked or "pegged" with near-size particles, which would impede slurry flow and could cause tank overflows. Furthermore, the installation of a final "carbon safety screen" on the tailings line is a critical best practice to capture any carbon that inadvertently escapes the primary circuit due to a screen failure.
The back-end circuit is the plant's main cost center, and its optimization is a key economic driver. This involves finding the optimal carbon advance rate, which determines how much Gold is loaded onto the carbon before it is sent for stripping. A higher loading reduces the tonnage of carbon that needs to be eluted and regenerated, resulting in significant savings in energy and reagent costs. However, this must be balanced against the risk of increasing soluble gold losses in the tailings, as a more heavily loaded carbon is less active. Process simulation models, such as SIMCIL, can be powerful tools for finding the economic sweet spot that balances these competing factors. Similarly, the regeneration kiln must be carefully controlled to ensure complete removal of organic foulants without overheating and physically damaging the carbon.
Table 4: Key Performance Indicators (KPIs) and Best Practices for Carbon Circuit Management | Parameter/KPI | Definition | Typical Target Range | Best Practice for Management/Optimization | |:--- |:--- |:--- |:--- | | Hardness (Ball Pan) | Resistance to physical degradation. | >98% | Select high-quality virgin carbon; optimize agitation to minimize attrition. | | Capacity (K-value) | Equilibrium gold loading at one ppm Au. | 25-30 kg/t | Select high-activity carbon; ensure effective regeneration to remove organic foulants. | | Kinetics (R-value) | Rate of gold adsorption in a set time. | 55-60% | Ensure effective acid washing to remove inorganic scale; maintain high carbon activity. | | Inorganic Fouling | Calcium carbonate (CaCO3) on carbon. | < 2% (post-wash) | Optimize acid wash cycle (acid strength, time); ensure good quality process water. | | Carbon Activity | Regenerated carbon activity vs. virgin. | 80-90% | Optimize regeneration kiln temperature and residence time; monitor organic foulants. | | Soluble Gold Loss | Dissolved Gold in final tailings solution. | < 0.01 mg/L | Maintain target carbon concentrations and activity profile across the adsorption circuit. | | Gold on Eluted Carbon | Residual Gold on carbon after stripping. | < 50 g/t | Optimize elution temperature, pressure, and reagent concentrations. |
The role of activated carbon in gold extraction is a definitive example of a successful, albeit complex, engineering trade-off. A clear economic imperative drove its development and widespread adoption: the need to profitably process vast reserves of low-grade and metallurgically challenging ores that were inaccessible to previous technologies. This has been an overwhelming success, underpinning the global gold industry for the last half-century.
The core of the trade-off lies in the technology's dual nature. The introduction of activated carbon into the ore slurry effectively addressed the critical problem of solid-liquid separation that had plagued the Merrill-Crowe process, enabling extremely high gold recoveries (>99%) from complex ore types, such as slimy and preg-robbing ores. This represents the "high efficiency" aspect of the technology. However, this front-end simplification was achieved by shifting the process complexity to a sophisticated and costly back-end circuit, which is required for elution, electrowinning, and thermal regeneration. This, combined with the persistent operational challenges of managing the carbon's physical and chemical degradation, represents the "high complexity" aspect. The successful management of a modern gold plant is therefore a continuous exercise in balancing these opposing characteristics to achieve an optimal economic outcome.
Despite its inherent disadvantages and the ongoing search for alternatives, carbon-based recovery via CIP, CIL, and CIC circuits remains the dominant and most widely applied technology in the global gold mining industry. For the majority of ore types encountered today, it remains the most robust, well-understood, and economically viable pathway for gold extraction. Its ability to handle a wide range of ore characteristics and its proven track record at massive scales have solidified its position as the industry's workhorse technology.
The future of gold hydrometallurgy is being shaped by research and development aimed directly at mitigating the known weaknesses of the activated carbon and cyanide-based paradigm. The direction of innovation is clear: to develop processes that are more selective, less energy-intensive, and more environmentally sustainable.
In conclusion, while activated carbon's position as the industry standard is secure for the near future, the technological landscape is evolving. The inherent challenges of the current process are serving as powerful catalysts for innovation, driving the industry towards a future of more efficient, selective, and sustainable gold extraction.

Ningxia Yongruida Carbon Co,.Ltd was founded in
2003.With an area of over 50000 square meters ,our
factory is located in the city of Shizuishan Ningxia .